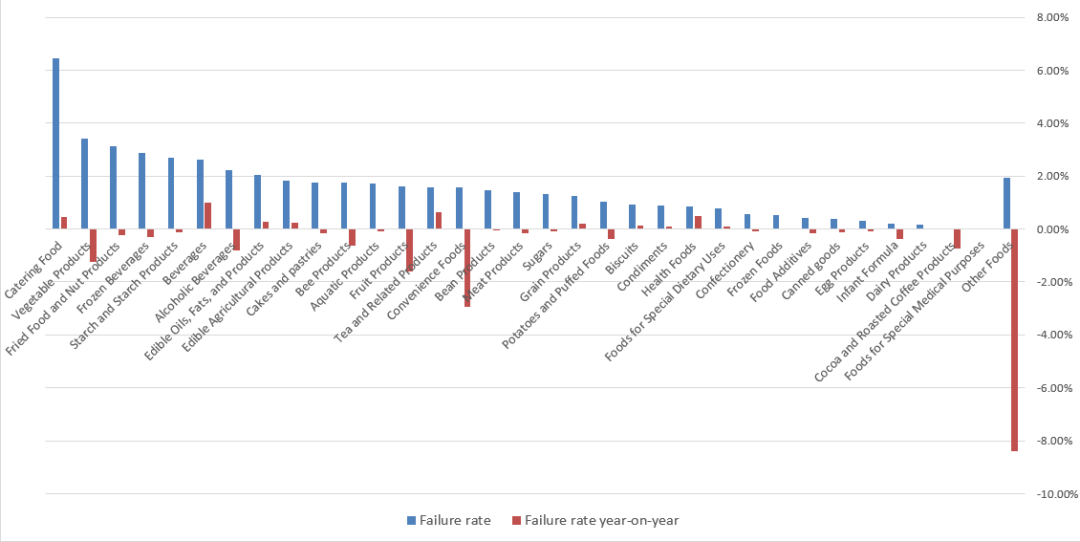
When the nutritional need of certain people cannot be met through ordinary meals or daily meals, FSMP can be used as an effective way of nutritional supplementation and play a role in nutritional support. The following is a brief description
Sodium Hyaluronate Can Be Used in General Foods
Fruiting body of Isaria cicadae Miq.(artificially cultured), sodium hyaluronate, and Lactobacillus kefiranofaciens subsp. kefiranofaciens, were approved.