To promote the work of filing nutrient supplement health foods, and in accordance with Food Safety Law of the People’s Republic of China, Measures for the Administration of the Registration and Recordation of Health Foods, and other relevant regulations and food safety standards, SAMR decided to revise Catalogue of Raw Materials of Health Foods (I) and Catalogue of Permitted Health Functions Claimed by Health Foods. Therefore, on November 29, SAMR issued the notice on soliciting public opinions on Catalogue of Raw materials of Nutrient Supplement Health Foods (Draft for Comments)(2019 Revision) and Catalogue of Permitted Health Function Claims by Nutrient Supplement Health Foods (Draft for Comments)(2019 Revision), the deadline for comments is December 31, 2019.
Antion will analyse the differences by the comparison between the current visions and Draft.
In the comparison of Catalogue of Raw Materials of Health Foods, the references of calcium acetate and calcium citrate are changed; compound sources and references of calcium, magnesium, iron, zinc, selenium, folic acid and choline are added in the Draft; and the nutrient “β-carotene” and its function “β-carotene supplement” are added in the Draft.
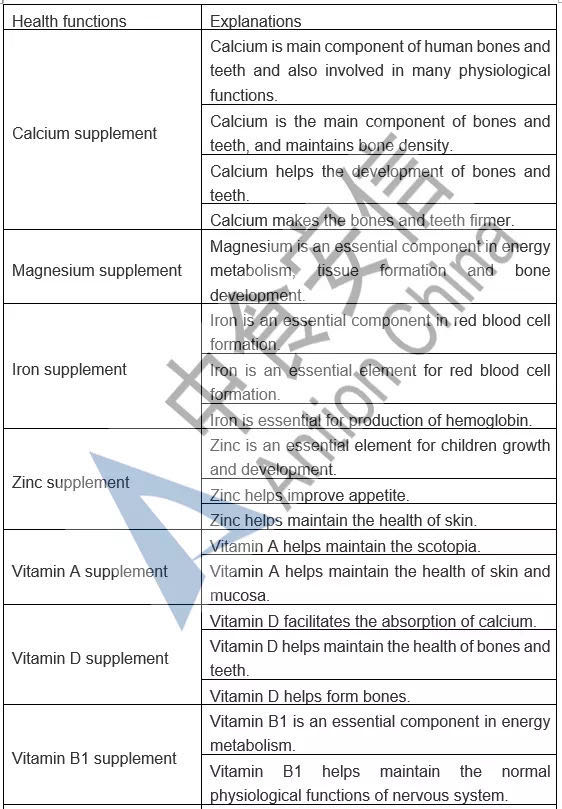
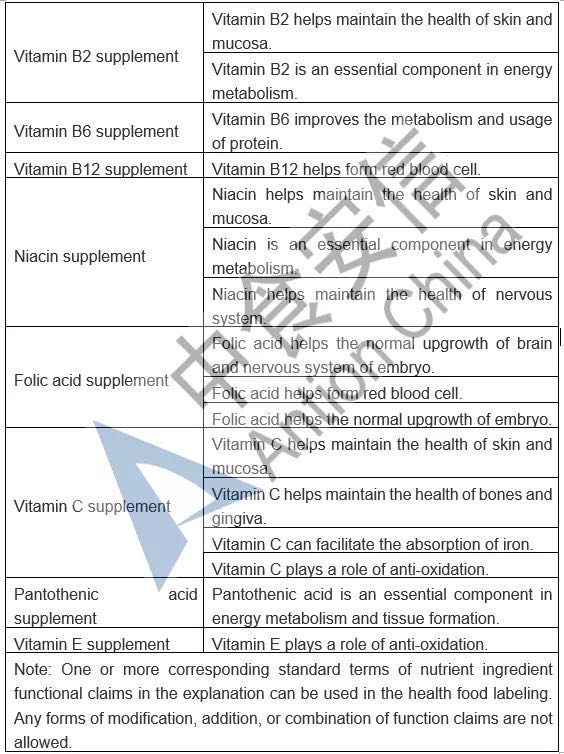
In the comparison of Catalogue of Permitted Health Foods Function Claims, it can be found that β-carotene is added in the Draft, not only that, the explanations of health function are added in the Draft are as follows: