In accordance with Food Safety Law of the People’s Republic of China, Measures for the Administration of the Registration and Filing of Health Foods, and other relevant regulations and food safety standards, State Administration for Market Regulation together with National Health Commission and National Administration of Traditional Chinese Medicine issued the Catalogue of Ingredients of Nutrient Supplement Health Foods (2020 Revision) and Catalogue of Permitted Health Function Claims by Nutrient Supplement Health Foods (2020 Revision), which will come into effect on March 1, 2021.
Antion will analyse the differences by the comparison between the previous version and 2020 Revision.
In the comparison of Catalogue of Health Food Ingredients (I), the references of calcium acetate, calcium citrate and zinc citrate are changed; compound sources and references of calcium, magnesium, iron, zinc, selenium, choline and pantothenic acid are added in the 2020 Revision; and the nutrient “β-carotene” and its function “β-carotene supplement” are added in the 2020 Revision. See table below for details:
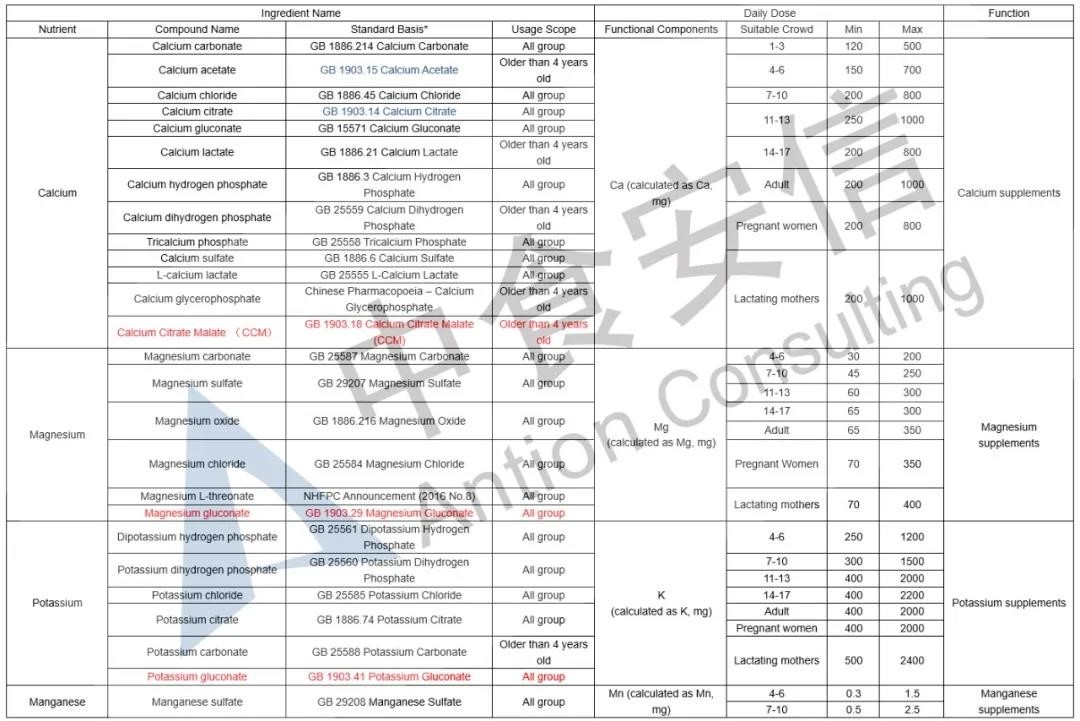
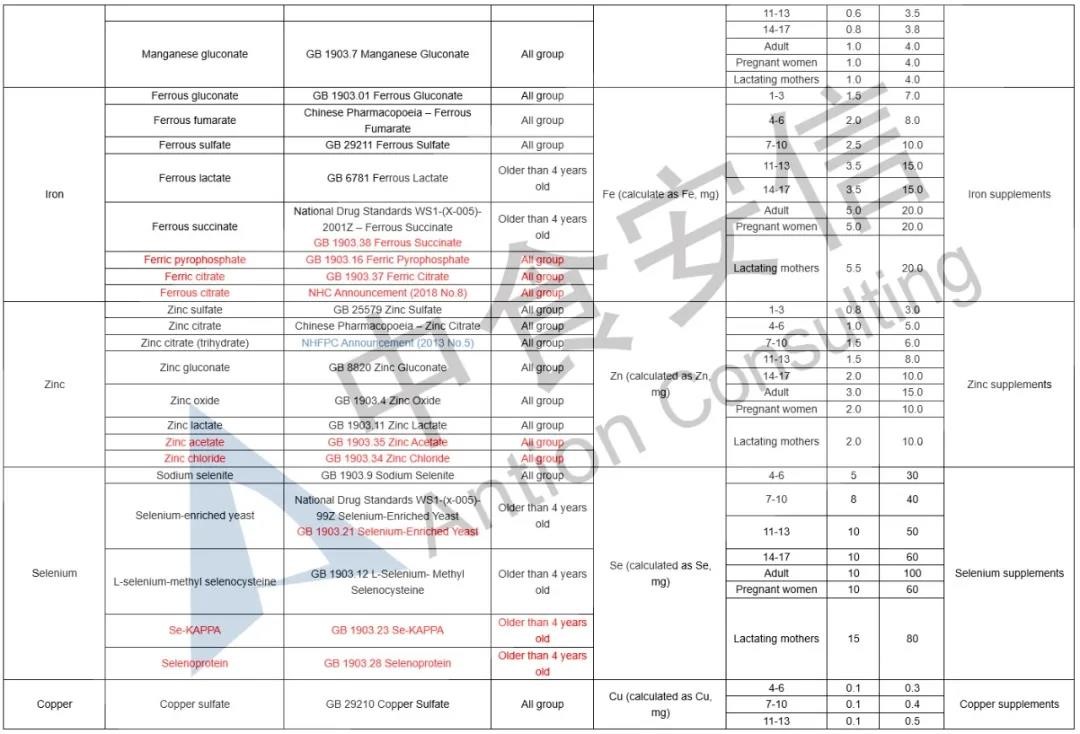
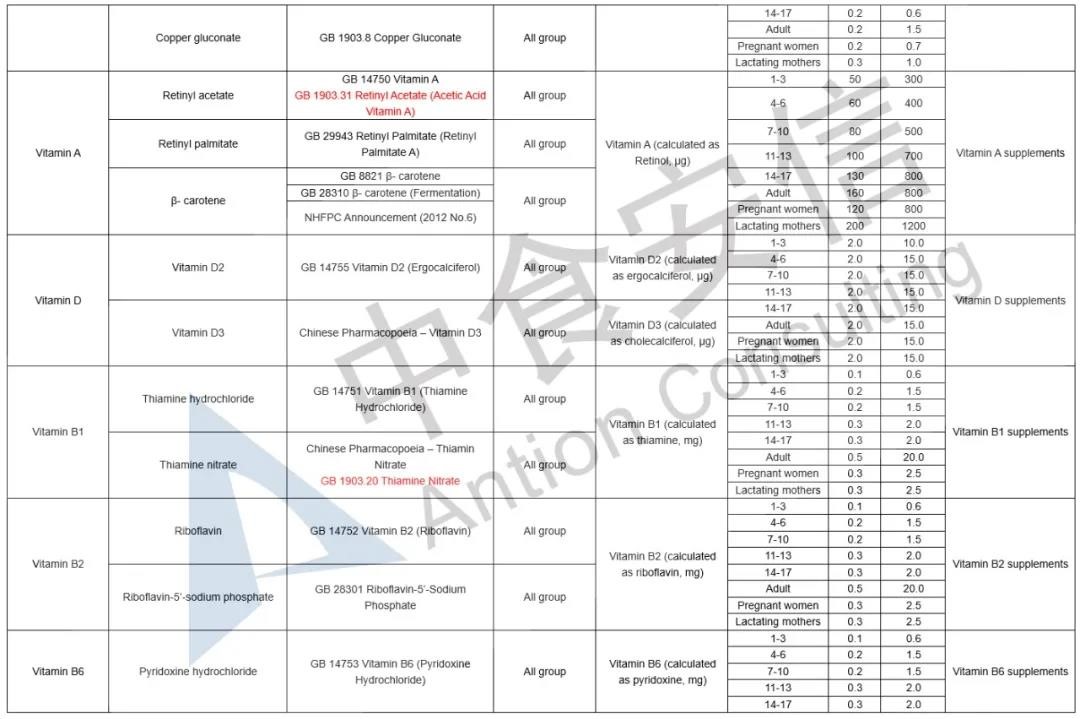
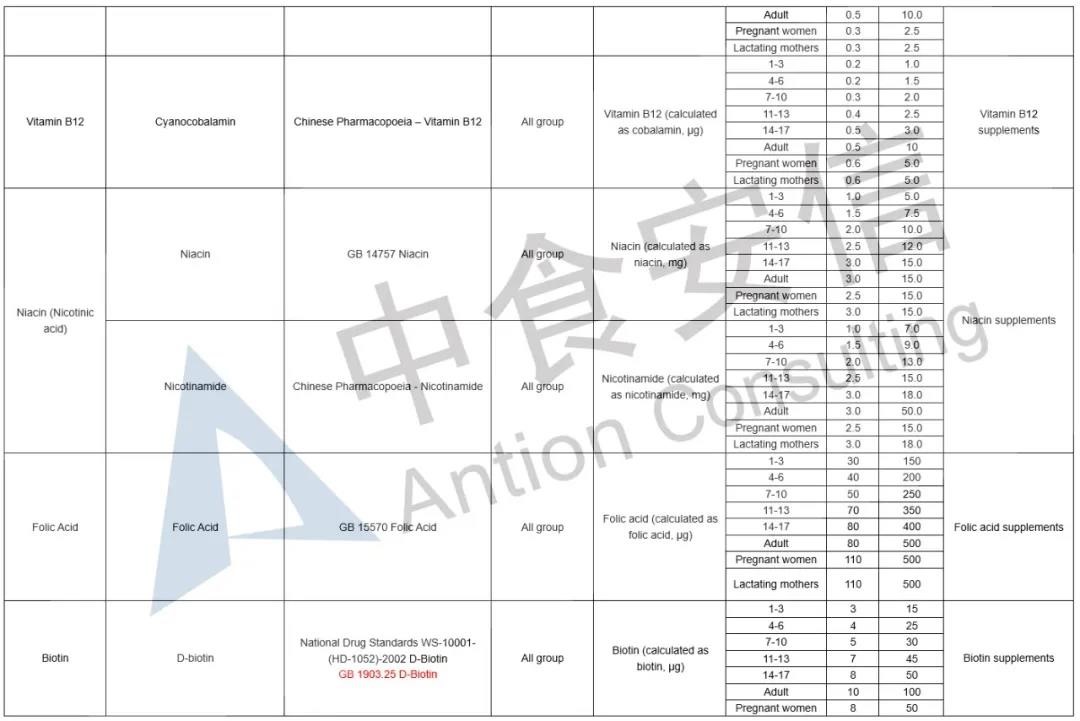
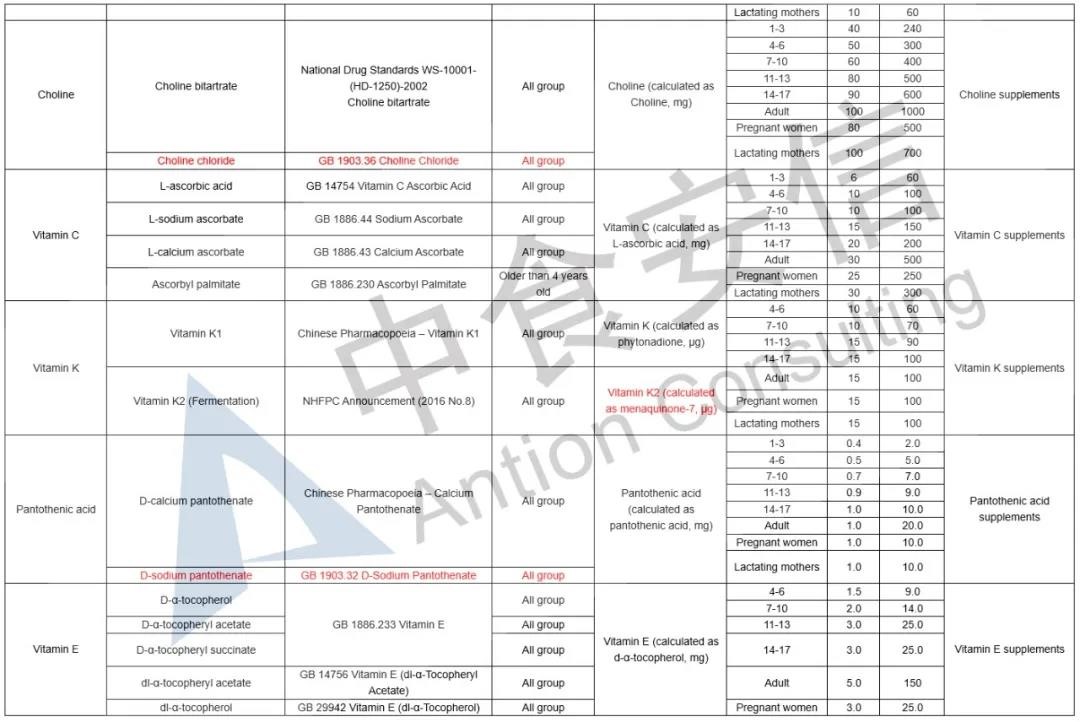
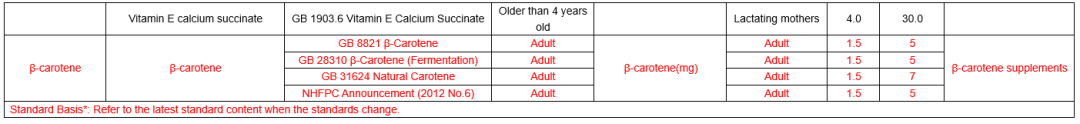
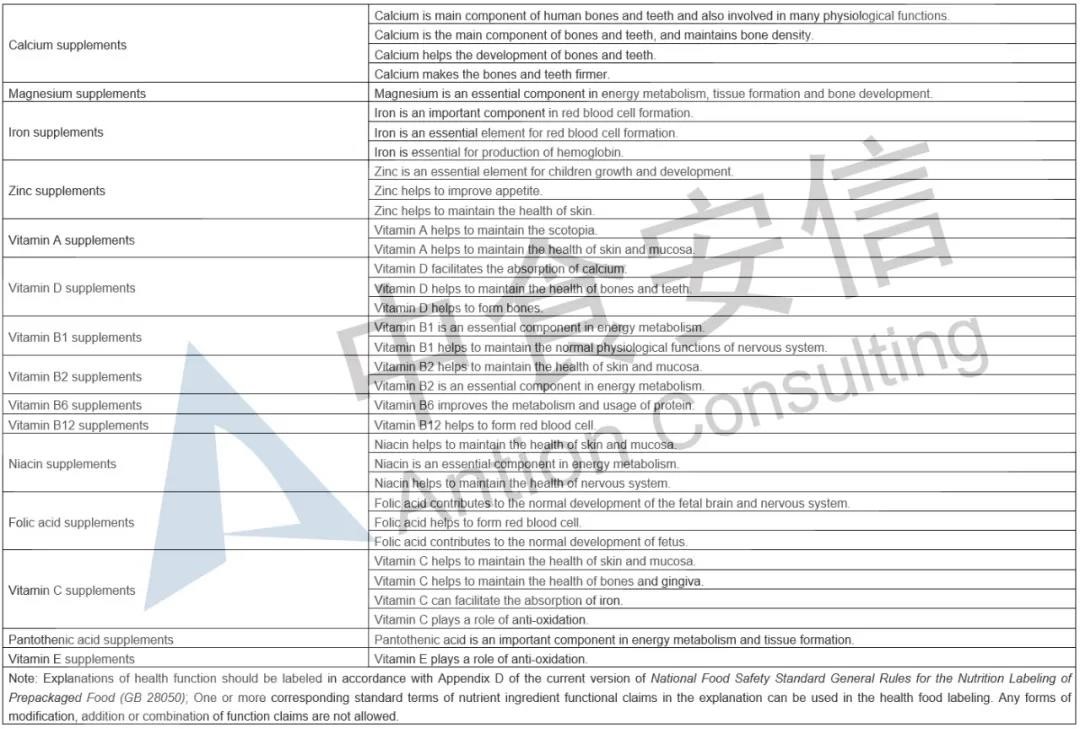
In the comparison of Catalogue of Permitted Health Foods Function Claims, it can be found that β-carotene is added in the 2020 Revision, not only that, the explanations of health function are added in the 2020 Revision are as follows:
More information about the Catalogues please click 【權威】《保健食品原料目錄 營養素補充(2020年版)》《允許保健食品聲稱的保健功能目錄 營養素補充劑(2020年版)》發布.