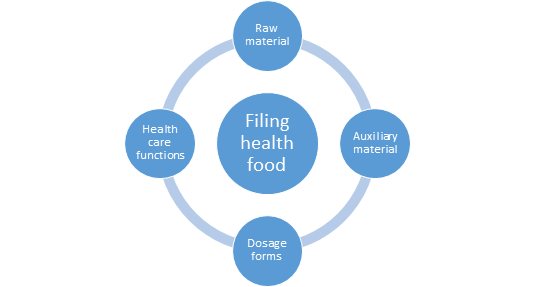
On December 8, 2021, China National Centre for Food Safety Risk Assessment (CFSA) publicly solicited opinions on a novel food ingredient Chlamydomonas reinhardtii. The deadline for comments is January 8, 2022. The two new varieties of enzyme
Q&A| Certification for R&D Capability of IF Formula Registration
What are included in the certification documents for research and development capabilities of formula registration of infant and young children formula milk powder?