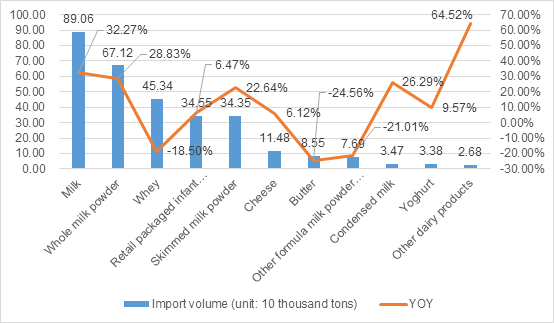
GACC recently announced the consumption data of infant formula milk powder imports in the first quarter, which shows that from January to March 2021, the domestic import of 61,000 tons of infant formula milk powder, down 17.6% compared to th
Differences between Probiotic Solid Beverage and Health Food
although both “live probiotic-type solid beverage” and “probiotic health food” are foods containing live probiotic, one is general food and the other is health food. They implement different management regulations and requirements an