The development of novel food ingredients is one of the important ways to innovate in the food industry, so the compliant use of novel food ingredients is also a problem that many food enterprises need to face when developing and innovating. In order to regulate the development and application of food raw materials and ensure food safety, the novel food ingredients must be applied and evaluated before entering the market. They can only be sold with the approval of the National Health Commission (hereinafter referred to as the NHC). Antion summarized the application of novel food ingredients during 2021, and conducted a brief trend analysis, hoping to be helpful to the use of raw materials or application plan of food enterprises.
Approval Status in 2021
For substances applied in the form of novel food ingredients, after evaluation, the NHC has two approval forms: 1) An announcement, reviewed, approved and announced in accordance with novel food ingredients; 2) Substances that are substantially equivalent or have a history of consumption in China, managed according to general food ingredients, that is, the review is terminated.
Approval Announcement in 2021
During 2021, there are 5 novel food ingredients approved by the NHC (divided according to the date of writing), namely β-1,3/α-1,3-glucan, dihydroquercetin, Lactobacillus rhamnosus MP108, Nannochloropsis gaditana and Rumexpatientia L. ×Rumextianshanicus A. Los. See the links below for details:
NHC| Six "Three Novel Foods" were Approved
A Food Flavoring Substance Applied by Antion has been Approved
Termination of Review in 2021
The termination of review will not be announced like the announcement of approval. The review results and opinions will be directly updated in the database of termination of review of the NHC. It is recommended that food enterprises pay attention to the update of the database at any time.
There are 11 raw materials terminated for review in 2021. See the table below for details:
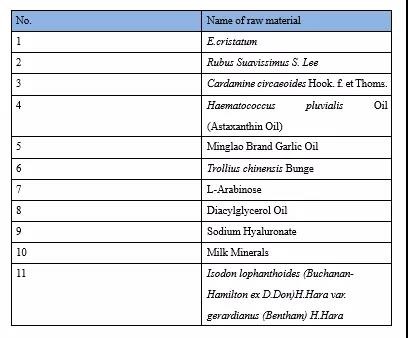
Trend Analysis
As many as 25 novel food ingredients was accepted by the NHC in 2021 (Appendix I). Judging from the current situation, as the only way for innovation in the food industry, combined with the background of the rapid development of functional foods in the post-epidemic era, the innovation and R&D of new ingredients are the focus of food or ingredient enterprises. However, with the increase in the number of applied raw materials, and the related regulations on novel food ingredients have not been updated for a long time, in order to more standardized and strict management, regulations on novel food ingredients may appear new versions or new rules supplemented in the next few years. It is recommended that enterprises preparing to apply novel food ingredients pay attention to related information at any time.
Judging from the situation of the five novel food ingredients approved by announcements in 2021, the biggest difference from the announcements in previous years is that the novel food ingredients with a small recommended consumption amount are required to be used in categories of use and usage in details. Although there are not many samples available for reference, the trend is also reflected in sodium hyaluronate approved at the end of 2020 and the draft of pyrroloquinoline quinone disodium (PQQ) salt being solicited for opinions (please see Appendix II for six raw materials for comments in 2021). It is recommended that food enterprises that are developing new raw materials refer to it as appropriate, and prepare related documents and evidence in advance, which will help improve the efficiency of applying for novel food ingredients.
Conclusion
The application and review of raw materials is the most strict and time consuming in "three novel food". Up to now, a total of 145 novel food ingredients have been approved and 65 have been terminated for review. In order to shorten the application cycle of raw materials, Antion recommends that enterprises make adequate preparations before application and submit complete application documents to improve application efficiency, so as to seize the leading position in the innovation trend of the food industry.
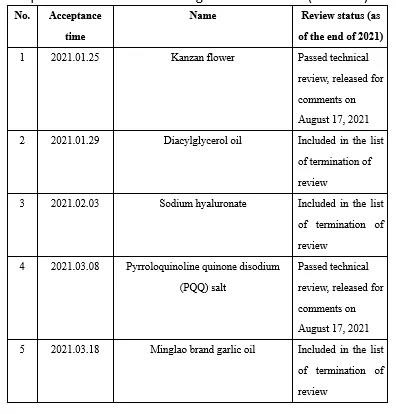
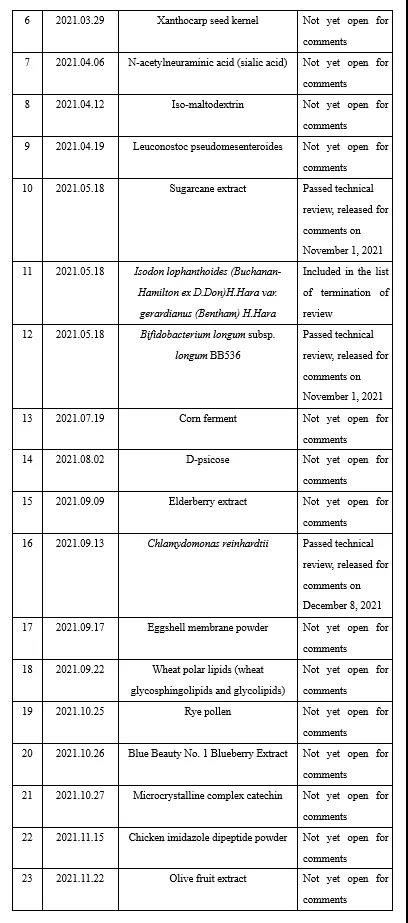

Appendix II Novel food ingredients issued by CFSA for comments in 2021 (6 kinds)
During 2021, CFSA issued six novel food ingredientss for comments, namely Lactobacillus acidophilus NCFM, Kanzan flower, pyrroloquinoline quinone disodium (PQQ) salt, sugarcane extract, Bifidobacterium longum subsp. longum BB536 and chlamydomonas reinhardtii. See the links below for details:
Two Novel Food Ingredients are Soliciting Opinions
CFSA| Soliciting Opinions on Two Novel Food Ingredients
CFSA| Soliciting Opinions on Three New Food
If you are interested in the application of novel food ingredients, please feel free to contact us!
Hongtao Fei
Tel: 010-51301566
email: feiht@antion.net
Source: Antion
Note: This article is compiled by Antion, please indicate our source if reprint it.