On December 1, 2020, the State Administration for Market Regulation, together with the National Health Commission and the National Administration of Traditional Chinese Medicine, released Catalogue of Health Food Ingredients Coenzyme Q10, Melatonin, Fish oil, Spirulina, and Sporoderm-broken of Ganoderma Lucidum spores powder, which will come into effect on March 1, 2021. This means that Coenzyme Q10, sporoderm-broken of Ganoderma Lucidum spores powder, spirulina, fish oil and melatonin can be declared as filing health foods from March 1 next year.
In order to better help enterprises to understand the relevant requirements, Antion analyzed and interpreted the main changes and effects in light of the current regulations.
Non-vitamin health food ingredients are added for the first time
On December 27, 2016, the former State Food and Drug Administration released the Catalogue of Health Food Ingredients (I) (hereinafter referred to as Catalogue). As the Catalogue only contains vitamin and mineral, enterprises are greatly restricted in the development and production of filing health food products. This is the first time since the implementation of the "dual-track system" for health food, that non-vitamin and mineral ingredients are added to the Catalogue, which has been the long-awaited thing in the industry. Therefore, the release of these five ingredients not only lays a foundation for the future expansion of other filing health food ingredients, but also promotes the rapid development of the health food industry.
Clear requirements on the use of relevant ingredients
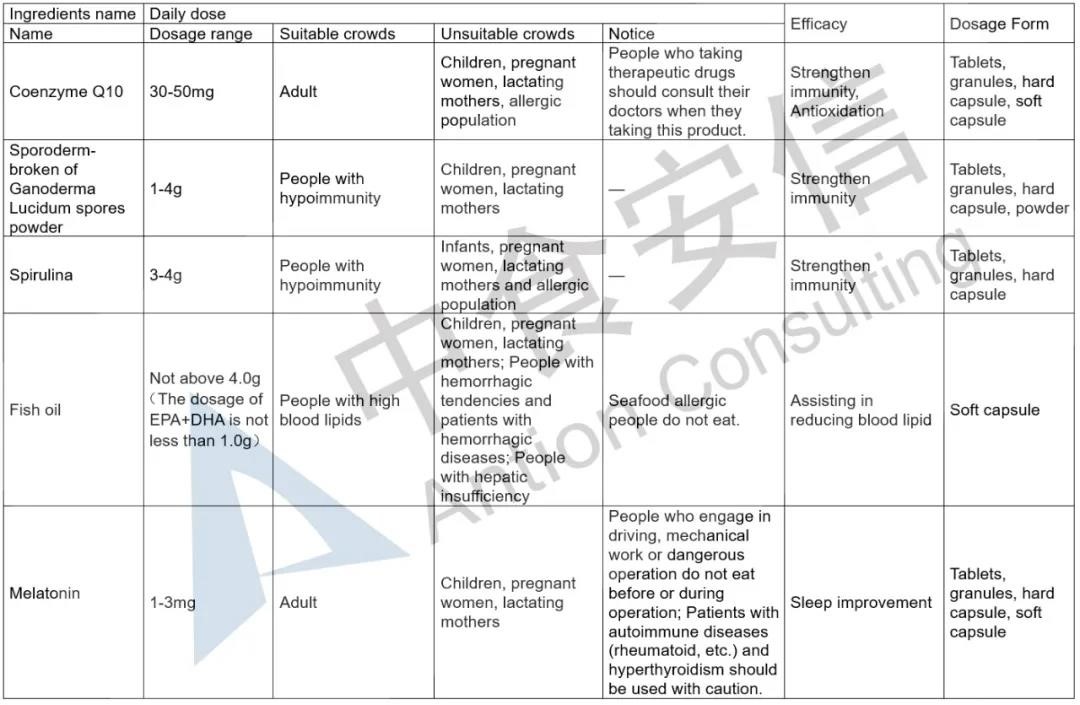
The announcement has detailed provisions on the dosage range, suitable crowds, unsuitable crowds, notice and efficacy of the five health food ingredients, and also makes clear requirements on the corresponding technical requirements of ingredients and product dosage forms. In order to make it easier for enterprises to read, Antion has sorted out the relevant basic requirements. In addition, it should be noted that, the five health food ingredients, except for melatonin which can be used together with vitamin B6, the other ingredients cannot be used together with any other health food ingredients in the Catalogue.
Expanded the functions of filing health food
Previously, the function of filing health food is only to supplement vitamins or minerals. The release of the five health food ingredients not only increases the ingredients of filing health food, but also clarifies the corresponding health food functions. This means that the function of non-supplementary nutrients is expanded on the basis of the existing filing health food function. For example, the health food function of Coenzyme Q10 is to enhance immunity and antioxidant.
It is noteworthy that the list of auxiliary ingredients available for the five health food ingredients was not released together, but on August 12, 2020, the State Administration for Market Regulation solicited opinions on the technical requirements of the health food ingredients, which is believed to be improved and released in the near future. Antion will continue to pay attention to this, please follow us.