In the first half of 2023, 24 FSMP products received registration approval, including 1 imported product. Notably, this period marked the first-time approval of a thickening component.
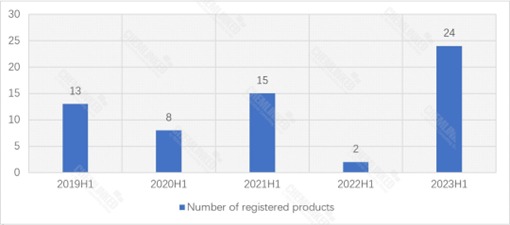
According to the data from Special Food Information Query Platform, 24 food for special medical purposes (FSMP) products were granted registration approval in 2023 H1. Among them, one imported product was approved:

From 2019 to 2023, 2023 H1 has seen the highest number of FSMP products gained approval, indicating that the State Administration for Market Regulation (SAMR) is prioritizing registration work this year.
China FSMP Registration in H1: 2019-2023
FSMP could be classified into products designed for infants (0-12 months) and products for people aged above 1 year. The latter type occupies the majority of approved products, with 20 gained approvals, compared with 4 approvals for the former type. Additionally, 2023 H1 saw the approval of the first thickening component, showing the diversity of registered FSMP types.
FSMP Products Approved for Registration in 2023 H1
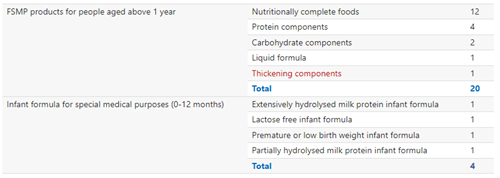
The FSMP types listed above are based on the GB standards related to FSMP products.
An overview of FSMP registration statistics
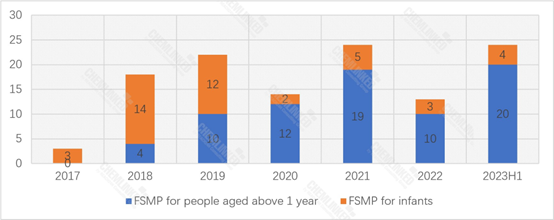
Up until June 30, 2023, China has approved 118 FSMP products. Despite only at halfway through the year, the number of registered FSMP products has already surpassed the annual totals of many previous years. Besides, since 2020, the annual registered FSMP products for people aged above 1 year has consistently surpassed those of infants.
FSMP Products Approved for Registration: 2017-2023H1
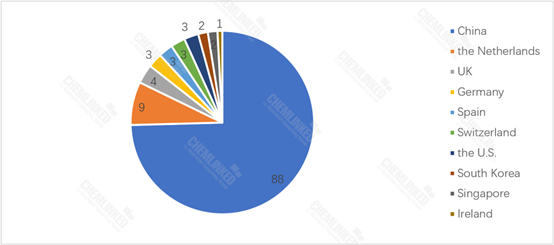
By the end of June, 2023, 30 imported FSMP products have been registered in China, with 9 originated from the Netherlands. Chinese products account for the majority of registered FSMP products, making up 74.6% of the total.
FSMP Products Approved for Registration: By Country
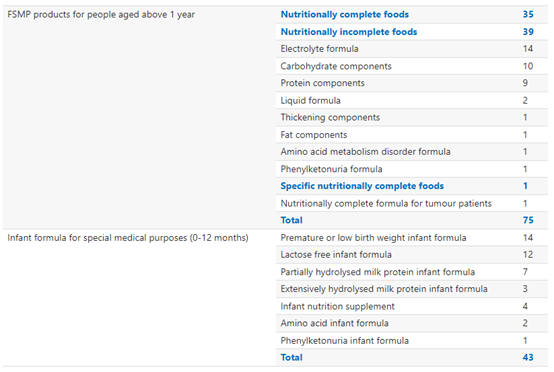
In total, 75 FSMP products for people aged above 1 year and 42 FSMP products for infants have received registration approval. Within registered FSMP products for people aged above 1 year, the most registered product type is nutritionally incomplete food. Within FSMP for infants, the most registered product type is premature or low birth weight infant formula.
All FSMP Products Historically Approved for Registration: By Product Type
In a recent reply to questions raised by the industry, SAMR states that it will expedite the revision of the Administrative Measures for Registration of Special Medical Purpose Formula Foods.
Notes:
Data are sourced from Special Food Information Query Platform. As the database may not provide real-time updates, there may be inconsistencies with the current regulatory situation.
Source: Chemlinked
Note: This article is compiled by Antion. Please indicate the source for reprint.